The prostate is an exocrine gland, whose secretion helps the survival and motility of sperms. It is also a major target for oncogenic transformation, as prostate cancer is the second leading cause of cancer death in men in America. The anatomy of the prostate organ is relatively simple. It is composed of multiple tubule-like prostatic ducts that contain epithelial and stromal compartments. The prostate epithelium contains just two layers of cells, namely, luminal cells that are responsible for protein secretion, and basal cells near the basement membrane. Rare neuroendocrine cells are also interspersed in the epithelium. The stromal compartment contains fibroblasts, muscle cells, and cells of the hematopoietic system. Almost all prostate cancer cases arise from the epithelium, but epithelial-stromal interactions play a critical role in both organ development and prostate cancer progression.
We are interested in tackling the following questions: What is the cell lineage relationship in the prostate? How do cross-talks among different cell populations regulate prostate normal homeostasis and disease progression? We have previously discovered the stem cell plasticity of basal cells, identified both basal and luminal cells as cells of origin for prostate cancer, and associated luminal-origin tumors with faster progression. Currently, we are taking the combined approach of genetic lineage tracing, organoid culture, biochemistry, single-cell RNA-seq, CRISPR genome-editing, and bioinformatics to investigate the following research topics.
Mechanism of basal stem cell plasticity in homeostasis and diseases
Basal-to-luminal cell differentiation is important for prostatic disease progression, since human prostate cancer predominantly show luminal phenotypes and lack of basal cells. Lineage-tracing analyses in mice showed that basal cells behave as adult stem cells to produce luminal cells during prostate organogenesis. However, in the mature prostate, basal-to-luminal differentiation is significantly restricted, but is reactivated under oncogenic or inflammatory conditions. Are mature basal cells under the inflammatory or oncogenic conditions reprogrammed to a state resembling the early postnatal basal stem cells? We are dissecting the internal and extrinsic signaling pathways such as Wnt and AR in regulating basal cell plasticity in normal homeostasis, prostatitis, and cancer initiation.
Roles of AR and Nkx3.1 in prostate stem cells
Androgen regulates numerous aspects of prostate physiology and diseases through the transcription factor androgen receptor (AR). Despite the use of androgen-deprivation therapy (ADT) in the clinics, prostate cancer almost always relapse after this treatment. This can be partially attributed to the distinct roles of AR in different cell types of the organ. For instance, whether AR in stromal cells is tumor-suppressing or -promoting is still under debate. Recently, our lab discovered cell-type-specific roles of AR in the prostate epithelium. In particular, AR was found to be cell-autonomously required for stem cell activities of basal stem cells as well as a type of luminal stem cell called CARNs. CARNs are distinguished by the expression of the gene Nkx3.1 in luminal cells after androgen deprivation. Being a transcriptional target gene of AR, Nkx3.1 encodes a key regulator of prostate cell fate specification and tumor suppressor. We are investigating the molecular mechanisms by which AR orchestrate different cell behaviors in prostate homeostasis and how Nkx3.1 is transcriptionally regulated.
Novel mouse bladder cancer models
The bladder is an urogenital system organ that shares many anatomical similarities with the prostate. Due to the complex mutational landscape of human bladder cancer, there is a relative lack of mouse bladder cancer models compared to many other cancers. With the support of an ACS grant, we are building novel bladder cancer mouse models via CRISPR, and are investigating the cell of origin for bladder cancer and the roles of epigenetic regulator genes.
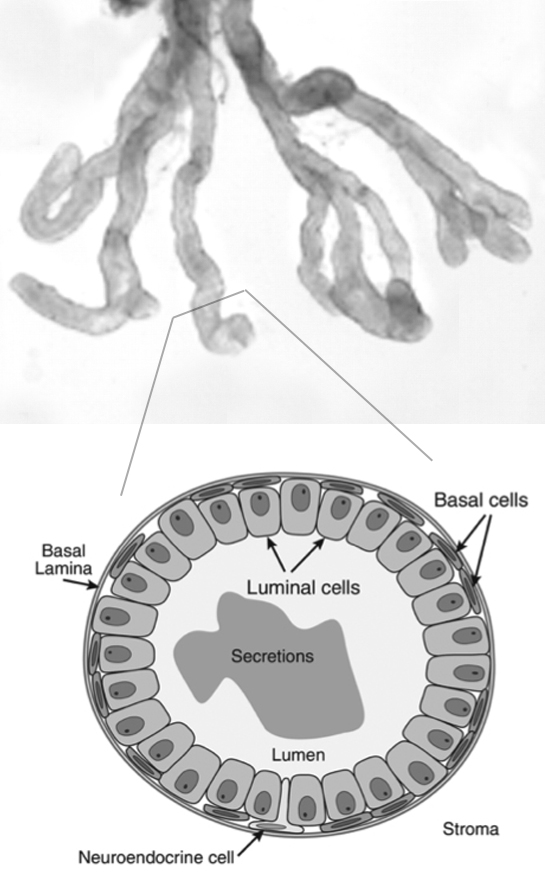
Prostate anatomy and cellular structure

Basal cell differentiation shown by lineage-tracing
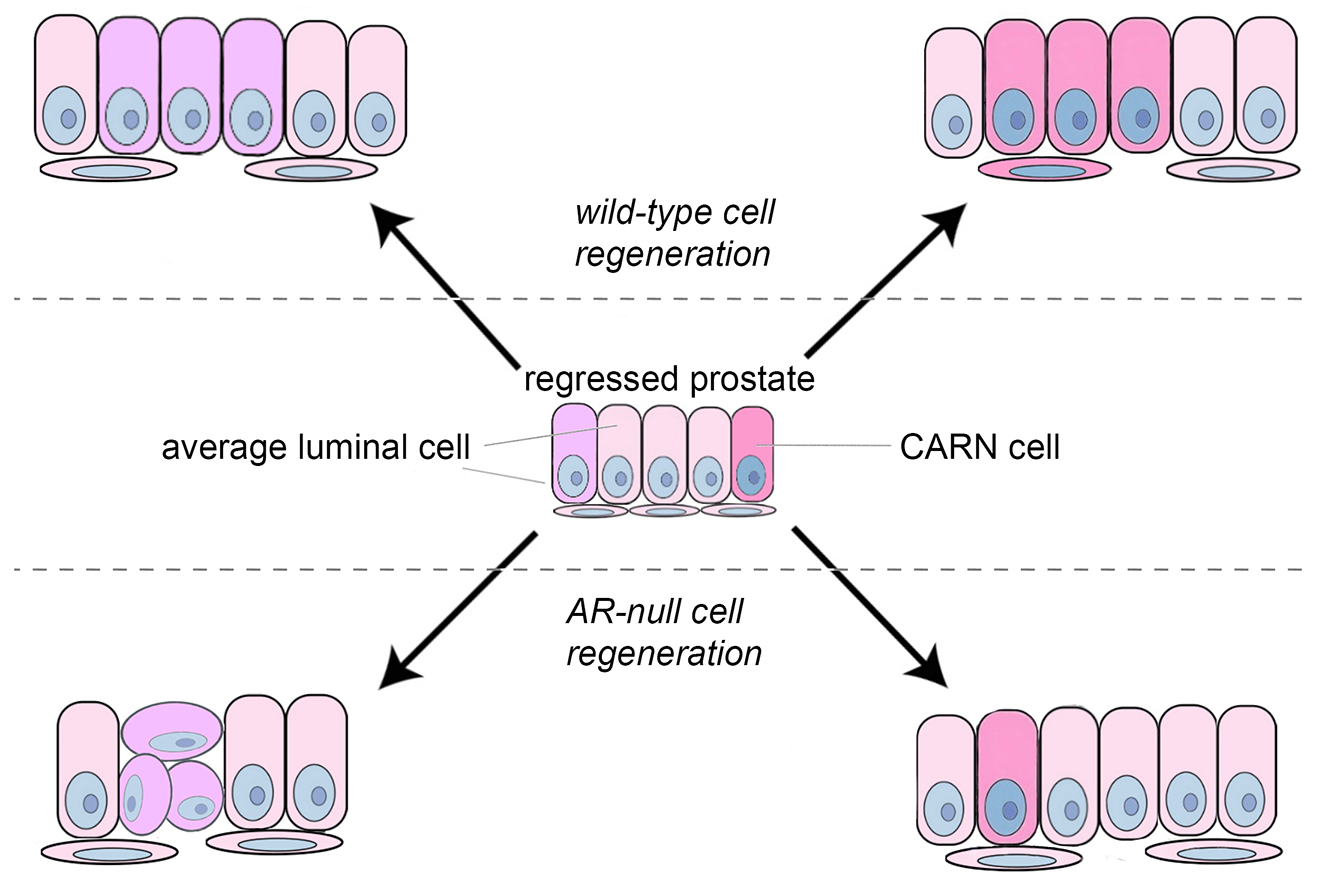
Model of AR's role in CARNs